- This topic is empty.
-
AuthorPosts
-
2025-10-11 at 3:11 pm #5236
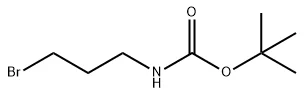
Tert-Butyl 3-bromopropylcarbamate (CAS No.: 83948-53-2), also known as N-Boc-3-aminopropyl bromide, is a vital compound widely used in the field of organic and pharmaceutical synthesis. It plays a key role as an intermediate for constructing complex molecules and serves as a protective reagent for amino groups in multi-step chemical reactions.
This compound is characterized by its molecular formula C8H16BrNO2 and molecular weight of 238.12 g/mol. It is typically observed as a white low-melting solid, with a melting point of 37–39 °C and a predicted boiling point of 285.3 ± 23.0 °C. With a density of 1.279 ± 0.06 g/cm³, it demonstrates stability under controlled storage conditions. In this blog post, SACH, a high purity industrial fine chemicals manufacturing factory, will share Tert-Butyl 3-bromopropylcarbamate in organic synthesis, its properties, synthesis methods, etc.
Physical and Chemical Properties of Tert-Butyl 3-bromopropylcarbamate
The distinct physical profile of Tert-Butyl 3-bromopropylcarbamate makes (CAS No.: 83948-53-2) it particularly attractive for laboratory and industrial applications.
* Appearance: White low-melting solid
* Molecular Formula: C8H16BrNO2
* Molecular Weight: 238.12 g/mol
* Melting Point: 37–39 °C
* Boiling Point: 285.3 ± 23.0 °C (Predicted)
* Density: 1.279 ± 0.06 g/cm³ (Predicted)
* Solubility: Limited solubility in chloroform and ethyl acetate
* Acidity Coefficient (pKa): 12.48 ± 0.46 (Predicted)
* Storage Conditions: 2–8 °C, dry and sealed environment
These parameters reflect its stability and controlled reactivity, which are crucial in precision-driven chemical syntheses.
Synthesis Methods for Tert-Butyl 3-bromopropylcarbamate
The preparation of Tert-Butyl 3-bromopropylcarbamate (CAS No.: 83948-53-2) can be achieved through different methods. Two commonly adopted approaches are:
1. Bromination Reaction Method
This method involves the bromination of 3-aminopropyl bromide using tert-butyl carbamate. It is widely valued for:
* Ease of operation
* Mild reaction conditions
* High yields
This makes it ideal not only for laboratory-scale synthesis but also for industrial-scale preparation, where reproducibility and efficiency are critical.
2. Carbon Tetrabromide Method
A more complex but efficient approach uses carbon tetrabromide (CBr4) and triphenylphosphine (PPh3) as bromination reagents. Here, 3-hydroxypropyl carbamate tert-butyl ester is treated in tetrahydrofuran (THF) at low temperatures. After staged additions and prolonged stirring, the crude product is purified via column chromatography, yielding N-Boc-3-aminopropyl bromide with high purity.
This method, while effective, requires costly reagents and careful operation, which may limit its use to specialized synthesis needs.
Applications of Tert-Butyl 3-bromopropylcarbamate in Research and Industry
Pharmaceutical Synthesis Intermediate
One of the primary uses of Tert-Butyl 3-bromopropylcarbamate is as an intermediate in drug development. Its Boc-protected amine functionality makes it an essential building block for PROTAC linkers and anti-tumor agents. The compound enhances drug stability, solubility, and bioavailability, contributing to advanced therapeutic design.
Organic Synthesis Reagent
Beyond pharmaceuticals, N-Boc-3-aminopropyl bromide functions as an alkylation reagent. It reacts with compounds containing active hydrogen, enabling the synthesis of diverse alkylated products. Additionally, its role as a protective reagent for amino groups ensures amine functionalities remain intact during subsequent synthetic transformations.
These properties make it indispensable in academic research, biotechnology, and industrial chemistry.
Handling and Safety Precautions of Tert-Butyl 3-bromopropylcarbamate
Like many brominated organic compounds, Tert-Butyl 3-bromopropylcarbamate (CAS No.: 83948-53-2) requires cautious handling.
* Hazard Classification: R22 – Harmful if swallowed
* Protective Measures: Avoid skin and eye contact; use gloves, lab coats, and goggles
* Storage: Keep in tightly sealed containers at 2–8 °C, away from heat sources and oxidants
* Reactivity: As a bromoalkane derivative, it may undergo nucleophilic substitution or elimination reactions under certain conditions
Researchers and operators must adhere to standard safety protocols to minimize risks during synthesis, transport, and application.
Market Perspective on Tert-Butyl 3-bromopropylcarbamate
With increasing demand in pharmaceutical intermediates and fine chemical reagents, the global market for compounds like Tert-Butyl 3-bromopropylcarbamate is expanding. Manufacturers often supply the product in 25 kg drums, maintaining a standard purity of 98% to meet industry requirements.
For bulk buyers, minimum order quantities often start from 1 kg, making it accessible for both small-scale research labs and large-scale production facilities. The balance between synthesis efficiency, reagent cost, and application diversity shapes the growing relevance of this compound in chemical supply chains.
Comparison of Synthesis Methods: Practical Insights
When choosing between bromination methods for producing Tert-Butyl 3-bromopropylcarbamate, several factors influence the decision:
* Bromination Reaction Method: Cost-effective, straightforward, and scalable for industrial settings.
* Carbon Tetrabromide Method: Higher purity yields, but expensive and time-intensive.
For most large-scale manufacturers, the first method provides a more practical balance between efficiency and yield. Conversely, the second method is often preferred in academic or specialized projects requiring ultra-high-purity intermediates.
With evolving methods for greener synthesis and improved cost-effectiveness, the compound’s role in the pharmaceutical pipeline is expected to strengthen.
Conclusion
Tert-Butyl 3-bromopropylcarbamate (CAS No.: 83948-53-2), also referred to as N-Boc-3-aminopropyl bromide, is a crucial intermediate in organic and pharmaceutical chemistry. With well-defined physical properties, versatile synthesis methods, and broad applications in drug and organic synthesis, it continues to be a compound of significant industrial and academic interest.
-
AuthorPosts
- You must be logged in to reply to this topic.